What Are Scientists Most Concerned About With Respect To Sea Turtle Conservation And Climate Change?
Abstract
Increasing incubation temperatures may threaten the viability of sea turtle populations. We explored opportunities for decreasing incubation temperatures at a Caribbean area rookery with farthermost female-biased hatchling production. To investigate the issue of artificial shading, temperatures were measured under uncomplicated materials (white sheet, white sand, palm leaves). To exam natural drivers of incubation temperature, temperatures were measured at average nest depths with shading on two beaches. Results from a pilot experiment suggest the near effective material was palm leaves. Shading decreased temperatures past a hateful of 0.60 °C (SE = 0.10 °C, Due north = 20). Variation between beaches averaged i.88 °C (SE = 0.xiii °C, Northward = twenty). We used long-term rookery data combined with experimental data to estimate the issue on sexual practice ratio: relocation and shading could shift ratios from current ranges (97–100% female) to lx–90% female person. A conservation mitigation matrix summarises our evidence that bogus shading and nest relocation are constructive, depression-cost, low-technology conservation strategies to mitigate impacts of climate warming for body of water turtles.
Introduction
Climatic change continues at unprecedented rates with well-documented impacts beyond terrestrial and marine environments, primarily affecting ecosystem function, species abundance and distributionsi. Climate change models signal an increment in temperature, sea level and precipitation2. These predictions are highly relevant to oviparous reptiles due to direct ecological threats affecting nest flooding, nesting site availability and suboptimal sex allocation in species with environmental sex determination3. Furthermore, population-scale consequences associated with increased temperatures arise in taxa exhibiting temperature-dependent sex determination (TSD), such as reptiles and some species of fishfour. Hence understanding how climatic change volition impact sex ratios and population viability in sea turtles, and how these impacts may be mitigated, have been identified as key bugfive.
Ocean turtles exhibit TSD with female hatchlings produced at higher temperatures, males at cooler temperatures and a counterbalanced sex ratio at the pivotal temperature (around 29 °C)half dozen. Higher temperatures not only increase female-biased sex ratios but also increase egg bloodshed, with these effects being consistent across populations and speciesvii,8. With the boilerplate global temperature predicted to increase by at least 2.6 °C by 2100nine, warming temperatures threaten sea turtles through effects on hatching sexual practice ratio skews and increased hatchling bloodshed10.
While in that location has been long-term business organization over the status of sea turtles, conservation efforts around the world have led to increases in nesting numbers for a wide range of populations and species11. Even so climate warming remains a threat for the viability of this group broadly and so assessing the effects of climate change was identified as a acme global research priority for successful hereafter conservation of turtles5 and quantification of the effect of warming temperatures is a conservation priority. Recent research efforts take focused on electric current and predicted hatchling sex ratiosseven with concerns that high female-biased primary sexual activity ratios are already common at most ocean turtle rookeries, exceeding 3:1 in >50% studies12. Many sea turtle rookeries are producing female person-biased populations (east.k., Brazilthirteen, Caribbean14,15, Florida16, Mediterranean17, Australia8), or will skew towards near feminisation of hatchling output within 50 years (e.one thousand. Australia18).
There are relatively few sea turtle rookeries where cooler sand temperatures produce counterbalanced hatchling sex ratios. Geographic locations at the latitudinal extreme of nesting ranges is an obvious driver of libation temperatures, for instance Southward Brazil and North Carolina in the Atlanticten,19. Natural beach variability and vegetation shading are important drivers for cooler temperatures on a remote Indian Body of water atoll20. Other studies have shown that coastal vegetation shading can beginning or filibuster climate modify driven female-biased primary sex ratios, e.grand., Guadeloupe, Caribbean area21. However, for sea turtle rookeries without coastal vegetation (eastward.g. Great Bulwark Reef islands in Australia, Ascension Island and some Cape Verdean islands in the Atlantic), the restricted natural variation in beach temperatures provides lilliputian or no resilience for a balanced primary sex ratio in futurity. This has led to the development of management interventions to conserve turtle populations, and options for decreasing incubation temperatures include bogus shading22, watering and relocation to greater sand depths23. While the general bear on of shading, increased depth and watering to absurd nests is well known, information technology is not known if the magnitude of these impacts is invariant beyond sites and hence whether they will provide the same touch on every bit a management intervention across the world. Hence farther trials of methods to artificially cool nests are needed.
The present study explores opportunities for decreasing incubation temperatures at a rookery with extreme female-biased hatchling production in St Eustatius, North East Caribbean. Recent incubation temperature studies suggested that iii species nesting at this rookery (leatherback turtle, Dermochelys coriacea, hawksbill turtle, Eretmochelys imbricata and dark-green turtle, Chelonia mydas) have had female-biased hatchling production for at least a century with <36% males produced every twelvemonthxiv. In this study, our aim was to examine the thermal effect of dissimilar shading techniques and consequent hatchling sexual activity ratio. (1) We investigate the well-nigh efficient low-technology, low-cost shading technique. (2) Informed by the results of shading experimentation, we examine the effect of environmental variables (beach, shade, depth) on temperature and hatchling sex ratio. (3) Using the results from the effect of different environmental variables, predict potential benefits of conservation actions and likely outcome for current and future chief sexual practice ratios at a rookery with extreme female bias.
Results
Assessment of shading techniques
Data were successfully retrieved from the six loggers on Zeelandia Beach. All loggers collected information for a period of 69 hours.
All 3 treatments were effective at cooling sand temperatures (Fig. ane). At the end of the experiment (i.e. after 69 hours) sand temperature recorded nether the white cotton canvass was 0.26 °C lower than the corresponding control temperature. Temperature recorded under the white sand was 0.28 °C lower than its respective control temperature, and temperature under the palm leaves was 0.40 °C lower than its corresponding control temperature.
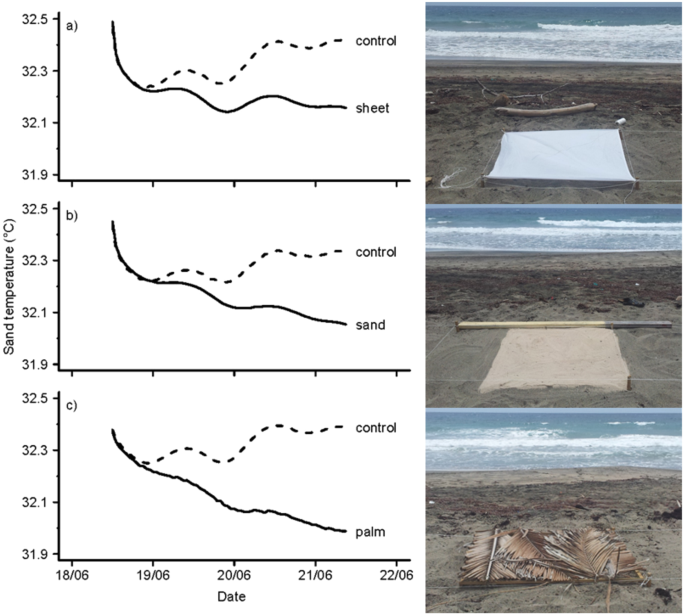
Iii different shading techniques were used to cool sand temperatures at mean hawksbill and light-green turtle nest depth (50 cm). The maximum difference between sand temperatures recorded under the white cotton canvass (a) and respective control temperatures was 0.26 °C. The maximum deviation between sand temperatures recorded under the white sand (b) and command temperatures was 0.28 °C. The maximum difference between sand temperatures recorded under the palm leaves (c) and control temperatures was 0.xl °C. This pilot experiment ran from 18 June 2012 until 21 June 2012 and provides a conservative approximate as the temperature differences between controls and shading treatments were expected to increment further after these 3 days.
To quantify the upshot of shading on temperature we calculated the difference betwixt sand temperatures recorded in a treatment plot (i.e. shaded with white cotton sheet, white sand or palm leaves) and sand temperatures recorded in the associated control plot. To reduce the result of temporal auto-correlation while retaining enough points for assay nosotros used 5-hour means in the analysis. An assay of covariance confirms that treatment had an effect on sand temperature (F5,36 = 367.3, p < 0.05, 95% Conviction Interval (CI) = −0.0355, −0.0308). While all iii treatments successfully lowered sand temperatures, palm leaves proved to be the most effective handling, reducing sand temperatures by approximately 0.3 °C during the first fifty hours of the experiment (F1,12 = 498.half-dozen, p < 0.05, CI = −0.0363, −0.0299). Using white sand as a treatment reduced sand temperatures by approximately 0.2 °C during the first 50 hours of the experiment (F1,12 = 476.7, p < 0.05, CI = −0.0268, −0.0219). Using the white cotton sheet also reduced sand temperatures past approximately 0.two °C during the beginning 50 hours of the experiment (F1,12 = 918.6, p < 0.05, CI = −0.0235, −0.0203). At the cease of 3 days, the deviation between temperatures under treatment plots and temperatures under the corresponding command plots continued to testify a linear and decreasing trend. Realistically, these differences should gradually attain horizontal asymptotes. Unfortunately our experiment did not run long plenty to reveal this. Regardless, the results from this brusque airplane pilot experiment informed with conviction which handling was most effective at cooling sand temperatures (see Fig. 1).
Effect of depth, shade and beach on sand temperature
Four loggers were lost due to beach erosion. The remaining xx loggers were successfully retrieved and included in the assay. Data from 26 November 2016 until 10 January 2017 were used for the analyses, covering a 46-solar day menstruation (Fig. 2).
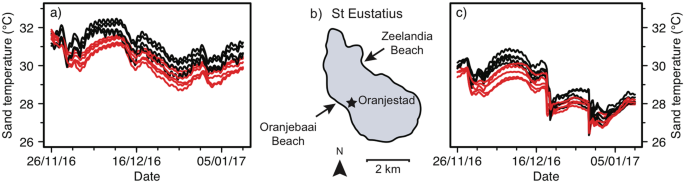
Sand temperatures recorded on St Eustatius between 26 November 2016 and 10 January 2017. A total of 20 temperature loggers recorded sand temperatures on (a) Oranjebaai Embankment (due north = four for command, n = 6 treatment) and (c) Zeelandia Embankment (n = five for control, due north = 5 treatment) in control plots (blackness lines) and plots shaded past palm leaves (red lines) at hateful turtle nest depth (l and 63 cm).
To study the relationship betwixt sand temperature, depth (i.due east. fifty vs 63 cm), treatment (i.eastward. control vs shaded) and beach (i.eastward. Oranjebaai Beach vs Zeelandia Beach) hateful sand temperatures were calculated for each logger. We performed a linear mixed model analysis to understand the relationship between sand temperature, depth, treatment and embankment. Depth, treatment and beach were entered every bit fixed effects. Site and plot were entered as random effects. Normality of the residuals was checked through visual inspection of the residuum plots. Likelihood Ratio Tests were used to obtain P-values. We tested the significance of the three-fashion and all two-way interactions by comparing a model with no interactions to a model with i interaction of involvement.
The main issue of beach on sand temperature was conspicuously visible in our data, with sand temperatures on Oranjebaai Beach being approximately 2.0 °C college than sand temperatures on Zeelandia Beach (Figs 2, iii). Similarly, the outcome of handling (command vs shaded) is clear, with shaded plots being approximately 0.half-dozen °C cooler than control plots (Figs 2, iii). In contrast, depth visibly shows no effect on sand temperatures (Fig. iii). The effect of shading increased past a cistron of ii (from approximately 0.3 °C to 0.6 °C) and results from the earlier shorter experiment are a conservative estimate. None of the interactions were meaning (χ2 = 5.27, df = 4, p = 0.26) so we used a model with no interactions. Our model confirms that beach had a meaning issue on sand temperature (χ2 = 21.34, df = 1, p < 0.05, CI = −ii.1801, −i.5885). Treatment as well had a meaning effect on sand temperatures (χ2 = 14.46, df = 1, p < 0.05, CI = −0.8433, −0.3637) while depth did non (χ2 = 1.66, df = 1, p = 0.20).
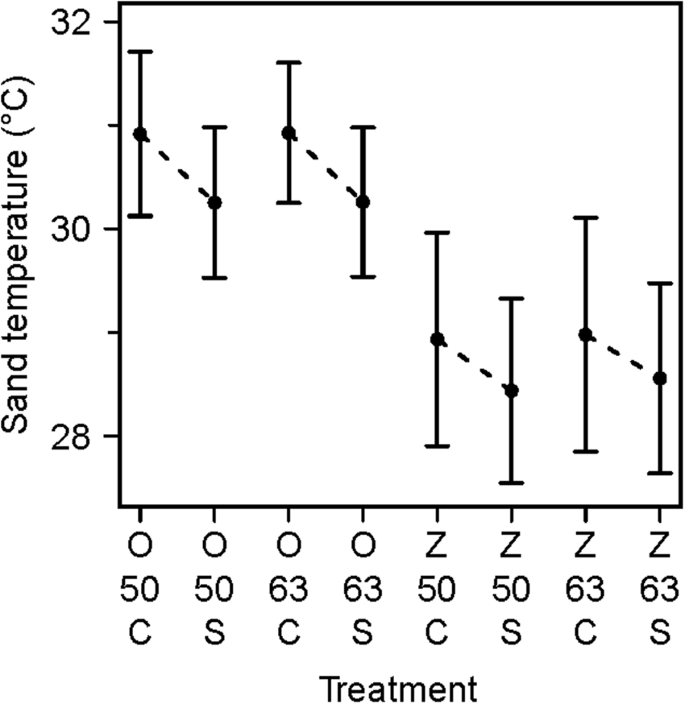
Beach and treatment had an outcome on sand temperature. Mean sand temperatures are given for each grouping of loggers in a treatment plot (i.e. shaded) and associated control plot. The intervals define the standard difference. O: Oranjebaai Beach; Z: Zeelandia Embankment; 50: l cm; 63: 63 cm depths; C: Control; S: Shaded. The dashed lines highlight the difference between a control plot and the corresponding shaded plot situated on the same embankment and at the same depth.
We constructed a conservation mitigation matrix relating temperature changes between written report beaches and treatments (Table 1). The biggest temperature divergence was institute between control plots on Oranjebaai Beach and shaded plots on Zeelandia Embankment (±2.487 °C).
Primary sex ratios
Mean monthly sand temperatures on Zeelandia Embankment are reported to vary from 29.1–29.6 °C in Jan–March to 31.9–33.3 °C in June–Baronial14. Using the equation describing the relationship between primary sex ratio and incubation temperature7, it is possible to approximate that the primary sex ratios are currently approximately 50–56% female-biased in Jan–March and 97–100% female person-biased in June–Baronial.
Lowering sand temperatures through the implementation of mitigation strategies such every bit relocating eggs from Oranjebaai Beach to Zeelandia Embankment and shading turtle nests would have an issue on sexual activity ratios (Tabular array 2). For example, a change of 1.0 °C would issue in primary sex ratios being approximately 21–34% female-biased during January–March and 91–98% female-biased in June–August. A subtract of 2.five °C would consequence in primary sexual activity ratios beingness approximately 4–7% female-biased during January–March and lx–90% female-biased in June–August.
It is projected that hateful incubation temperatures on St Eustatius will increment by approximately 1.1 °C by the year 2030, by 2.0 °C by 2060 and by 3.2 °C past 209014. Without mitigation strategies, this will event in primary sex ratios beingness shut to 100% female by the end of the century. Nest relocation or artificial shading would only have a measurable result on master sex ratios during January–March (Table 2).
Give-and-take
Our results demonstrate that simple, low price, low technology artificial shading can have a significant impact on ocean turtle incubation atmospheric condition. All shading strategies reduced temperature and the most effective treatment was palm leaves, supporting results of previous studies24,25,26. This contrasts to a previous shading experiment (using solar wave fabric) that had variable results27. Furthermore, we written report that relocation to a libation windward beach can reduce the temperature of the eggs by a cistron of four. We calculated the effects of these simple conservation actions, which would result in a positive shift from a female-skewed to a balanced primary sex ratio in the current conditions. The application of our results to mitigation strategies provides grounds for cautious optimism that unproblematic and low-cost conservation actions tin can be constructive at rookeries with high incubation temperatures and female-skewed primary sex activity ratios.
The effects in temperature decrease due to artificial shading at our study site are comparable to the magnitude of furnishings of shading by coastal vegetation and wood border on naturally shaded nesting beaches20,21. This suggests that artificial shading tin exist a substitute for natural vegetation shading at sea turtle rookeries in arid environments (e.g. Keen Barrier Reef islands). Depth does not accept such a clear issue as shading and differs due to a delayed time-lag response during periods of cooling and warming as well as a reduced pattern of diel variation at depthfourteen,20. Relocation of nests to different depths that reflect depths of natural nests at the same beach may not take the anticipated results because of complicated interactions between depth and the surround. Notwithstanding, our experiments were not designed to look at the full extent of depth impacts, since nosotros merely varied the range of depths adequately minimally (fifty–63 cm) respective to the hateful natural depth of body of water turtle nests on the island, while the difference betwixt the minimum and maximum depth of incubating eggs on the report beach is likely to accept a much greater effect. Although relocation practices tin can negatively touch egg and hatchling survivorship28, examples of successful relocation conservation programmes exist worldwide29,30. Our results further demonstrate that shading in combination with relocation to a different cooler beach can take much greater benefits for a balanced chief sex ratio.
While results from experiments studying the consequence of incubation temperature on emergence success have been inconclusive for St Eustatius14, information technology is possible to employ published relationships to estimate the impact mitigation strategies would have on emergence successes. For case, at an incubation temperature of 32.five °C, emergence success is likely to be <50%vii. Lowering temperatures by two °C through mitigation strategies (eastward.k. relocation) could increase the emergence success to >eighty%. A further 0.v °C reduction in incubation temperature (e.g. shading) would bring emergence successes close to 85%. This serves to illustrate the bear upon mitigation strategies can have on both primary sex activity ratios and emergence successes of bounding main turtles.
The variation in thermal ecology of two nesting beaches (<1 km distance) at our study site was surprising equally the sand on both beaches is of similar (volcanic) origin and inside-beach variation was non significant in a previous study14. The strong northeastern trade winds31 are likely to provide a cooling consequence for Zeelandia Beach. At this cooler beach, temperatures are low enough to produce a balanced primary sex ratio during off-peak nesting flavor in electric current weather condition. At that place is show that turtles select particular micro-habitats for nesting. For example, hawksbill turtles nest nether vegetation32, loggerheads Caretta caretta nest on mangrove islands33 and painted turtles Chrysemys picta seek shaded nest locations at warmer sites34. Protection of rookeries that announced minor in terms of numbers may exist essential for the health of the larger regional populationnineteen. Similarly, beaches with a range of shade cover should be identified to allow plasticity in nest-site choice21. Information technology is important to accept a regional approach to assess temperature at all rookeries and identify high priority cool beaches for protection.
When projecting into the future, warming air temperatures are predicted to cause the mean incubation temperatures at our report site to reach 32.ane °C by 2030 and 34.2 °C by 209014, above the upper thermal limit of 33 °C for successful incubation35. Alongside warming air temperatures, global body of water level ascension will inundate beaches and limit available nesting habitat36. In the meantime, these temperatures will exacerbate existing female person bias: female person green turtle sex ratios accept been >95% since 2009 and female sexual practice ratios of leatherbacks and hawksbills are projected to reach >95% past the years 2028 and 2045 respectively14. A key result remains whether phenological shifts in the seasonal timing of nesting volition help mitigate predicted impacts of climate warming. In the absence of phenological shifts in nesting or conservation actions, information technology is possible that this rookery volition no longer support viable nesting turtle populations. However, we urge caution with extending these conclusions to other areas since our results are from a rookery with extreme sand temperatures. Relocating and shading of nests at other rookeries is likely to produce unlike results than those reported in this report, then we emphasize that our results should be perceived every bit guidelines but. A vulnerability assessment framework37, microclimate model38 or sensitivity map39 may assistance with gaining a thorough understanding of natural variability of beach temperatures before planning conservation strategies such as relocation or artificial shading. A farther outcome for consideration is the practicality of management interventions, i.e. how to scale upward cooling treatments of a few days for a handful of sites upwardly to 100 s or g s of nests over several months.
With incubation temperatures projected to rising by up to 4 °C over the adjacent century at this study site14, these mitigation strategies may prove essential to guarantee that future operational sex ratios and emergence successes are feasible. However, the maximum temperature decrease achievable by relocation and artificial shading is less than this predicted temperature increase, and so will only exist sufficient to maintain a counterbalanced master sex ratio in the cooler off-meridian nesting seasons. Our model is limited by the apply of estimated values for master sexual practice ratio and emergence success. As shown for other rookeries, information technology is possible that male embryo survival and different male person and female breeding periodicities volition mitigate the highly female person-skewed chief sex activity ratio7,12. In light of new enquiry that demonstrates that higher temperatures increase the natural growth charge per unit of turtle populations (as more than females are produced) but that long-term population survival is threatened (once incubation temperatures near lethal levels), an improved agreement of emergence success may exist more than of a priority than sex ratios40. Finally, if sex ratios of a minor and endangered population are significantly skewed, there may be consideration of a captive breeding programme (e.one thousand. a hatchery) to release additional individuals into the wild to back up small or failing populations41 or an artificial modify in the weather condition at the egg-laying site42. The sudden drops in temperature nosotros attributed to rainfall accept besides been noted elsewhere on nesting beaches43. This bear on of rain on nest temperatures highlights how the impacts of changing rainfall patterns also need to exist considered when assessing the affect of climatic change on sea turtle hatchling sex ratios.
Constructive transboundary conservation of migratory species is a hard claiming faced by many conservation bodies44. Direction of nesting beaches is a widespread practice for endangered turtle populations45. Although results from our study contribute to a greater understanding of conservation strategies to decrease incubation temperature, in that location is a consensus that greater understanding of risks and effectiveness associated with conservation programmes is required5. Several regional sea turtle conservation networks exist, e.g., Wider Caribbean Sea Turtle Network (WIDECAST; world wide web.widecast.org) and the Indian Ocean–Southeast Asian Memorandum of Understanding for the Conservation and Management of marine turtles and their habitats (www.ioseaturtles.org). These networks may serve equally starting points to identify high priority absurd beaches for protection and for discussions near a standardised framework for embankment conservation actions at rookeries with farthermost female-biased primary sexual practice ratios and that lack natural coastal vegetation to heighten natural resilience in the face of global climate change46.
Methods
Study site
The 21 km2 island of St Eustatius is located in the Lesser Antilles in the n-eastern Caribbean. Almost all clutches are laid past leatherback, hawksbill and green turtles on 2 beaches: Oranjebaai Embankment (sheltered, western declension; 17.483°N, 62.988°W) and Zeelandia Beach (exposed, eastern coast; 17.507°North, 62.980°W), the latter beingness the nigh important nesting embankment46. The typical nesting season is March until June (leatherback), June until November (hawksbill) and July until October (green) (JB, NE, STENAPA unpublished data). The study was conducted inside the Statia National Marine Park programme and complied with all relevant local and national legislation.
Temperature loggers
Tinytag Plus 2 loggers (Tinytag Plus two model TGP-4017, Gemini Information Loggers, UK) were used to record sand temperature at depths representative of nests for leatherback, hawksbill and greenish turtles nesting on Oranjebaai Embankment and Zeelandia Embankment during 2012-2013 and 2016-2017. Temperature measurements were recorded every hour. The loggers were originally calibrated to United kingdom Accreditation Service (UKAS) standards and are accurate to <0.5 °C (world wide web.tinytag.info, last accessed on 28 March 2018). To minimize impact on natural conditions during burial of loggers, care was taken to excavate a sand core and and then supersede it back on top of the logger. This was achieved by hammering a 10 cm diameter PVC pipe to the desired depth of the logger, creating a vacuum and then removing the pipe full of sand. The depth of the pigsty was verified with a semi-rigid tape measure, then the logger was dropped into the hole and the sand was emptied out of the pipe on tiptop of the logger. A cord was connected to the logger to facilitate relocation of the loggers. GPS positions of the loggers were recorded. Subsequently burial, one twenty-four hour period was allowed for the sand temperatures to return to original country later disturbance. To ensure all loggers were exposed to the aforementioned caste, the surface of the experimental area was cleared from seaweed patches, other organic material and debris.
Assessment of shading techniques
An bogus shading experiment to examine the furnishings of low-price and bachelor shade materials on sand temperatures was conducted over a 72 hour period from 18–22 June 2012 on the principal turtle nesting beach, Zeelandia Beach. Loggers were deployed at mean nest depth of 50 cm (hawksbill and green turtles; JB, NE, STENAPA unpublished information). Three shading materials were used: white cotton sheet, white (imported) sand and (local) palm leaves (Cocos nucifera) (Fig. 1). An surface area of one.v × 10 m was cleared (i.e. organic textile and droppings were removed) and the sand surface was raked flat. Six loggers were cached in a row parallel to the waterline at one.5 m intervals and 50 cm depth. After burying loggers, bogus shading methods (ane m² surface area) were immediately placed on the sand surface so that each logger was centrally located within the experimental plot.
Consequence of depth, shade and embankment on sand temperature
Loggers were buried at mean nest depths of fifty cm (hawksbill and green turtles) and 63 cm (leatherback turtles). Hateful depths were calculated from records as the midpoint between the top and lesser of clutches of eggs excavated between 2005–2015 (JB, NE, STENAPA unpublished information). To examine the fundamental drivers of sand temperature we measured the touch of shading and nest depth at 2 beaches: Oranjebaai Embankment (Southwest coast) and Zeelandia Beach (Northeast declension). At each beach iii different plots were selected equally replicates. In total, 24 loggers were buried: 12 were buried at each site (beach); at each site 4 were buried in each plot (replicates); within each plot 2 were buried for each treatment (control vs shaded); within each handling 1 logger was cached at each depth (50 or 63 cm). The experiments were conducted from 23/09/2016–22/11/2016 and from 25/11/2016 until 09/01/2017. Initially the Oranjebaai Beach experiment was at the north end. A heavy storm on 17/eleven/2016 eroded the embankment, several loggers were lost and loggers were relocated (using the same treatments) to the south end of Oranjebaai Embankment. Shaded areas were created using palm leaves attached to 1 m² wooden frames with biodegradable cotton string and placed over the buried loggers. The wooden frames were made using branches from small native trees. The palm leaves were collected from the ground next to palm trees effectually St Eustatius.
Data were downloaded from temperature loggers using TinyTag Explorer 4.7. Prior to the analyses, information from before logger deployments, during relocation periods, and from after retrievals were discarded. All datasets were reviewed and checked for anomalies. Nosotros used R47 and package lme448 for analyses.
Chief sex ratios
The relationship betwixt incubation temperature and primary sex ratio7 was used to guess the potential that mitigation strategies have to touch on sex ratios at this field site. We used this model to calculate the expected change in primary sexual practice ratios with cooling regimes of 1 °C, 2 °C and ii.5 °C.
References
-
Jones, M. C. & Cheung, W. W. 50. Multi-model ensemble projects of climatic change effects on global marine biodiversity. ICES J. Mar. Sci. 72, 741–752, https://doi.org/10.1093/icesjms/fsu172 (2015).
-
Easterling, D. R. et al. Climate extremes: observations, modelling, and impacts. Science 289, 2068–2074, https://doi.org/10.1126/scientific discipline.289.5487.2068 (2000).
-
Mainwaring, Thou. C. et al. Climatic change and nesting behaviour in vertebrates: a review of the ecological threats and potential for adaptive responses. Biol. Rev. 92, 1991–2002, https://doi.org/10.1111/brv.12317 (2017).
-
Somero, One thousand. N. The physiology of climatic change: how potentials for acclimatization and genetic adaptation will make up one's mind 'winners' and 'losers'. J. Exp. Biol. 213, 912–920, https://doi.org/10.1242/jeb.037473 (2010).
-
Rees, A. F. et al. Are nosotros working towards global research priorities for management and conservation of sea turtles? Endanger. Species Res. 31, 337–382, https://doi.org/10.3354/esr00801 (2016).
-
Ackerman, R. A. The nest environment and the embryonic development of sea turtles. Pages 83–106 in Lutz, P. L. & Musick, J. A. (editors), The Biology of Bounding main Turtles, Volume I. CRC Press, Boca Raton, Florida, USA (1997).
-
Hays, Yard. C., Mazaris, A. D., Schofield, G. & Laloë, J.-O. Population viability at extreme sex activity-ratio skews produced by temperature-dependent sexual activity determination. Proc. R. Soc. B. 284, 20162576, https://doi.org/10.1098/rspb.2016.2576 (2017).
-
Jensen, One thousand. P. et al. Environmental warming and feminization of ane of the largest ocean turtle populations in the world. Curr. Biol. 28, 154–159, https://doi.org/ten.1016/j.cub.2017.11.057 (2018).
-
IPCC. Climate change 2014: Synthesis Report. Contribution of Working Groups I, 2 and Iii to the Fifth Cess Written report of the Intergovernmental Panel on Climatic change [Cadre Writing Team, Pachauri, R. K. and Meyer, L. A. (eds)]. IPCC, Geneva, Switzerland, 151 pp (2014).
-
Hawkes, L. A., Broderick, A. C., Godfrey, M. H. & Godley, B. J. Investigating the potential impacts of climate change on a marine turtle population. Global Change Biol. 13, 923–932, https://doi.org/10.1111/j.1365-2486.2007.01320.x (2007).
-
Mazaris, A. D., Schofield, Chiliad., Gkazinou, C., Almpanidou, V. & Hays, Chiliad. C. Global ocean turtle conservation successes. Sci. Adv. 3, e1600730, https://doi.org/x.1126/sciadv.1600730 (2017).
-
Hays, G. C., Mazaris, A. D. & Schofield, K. Different male person vs. female breeding periodicity helps mitigate offspring sex ratio skews in bounding main turtles. Forepart. Mar. Sci. 1, 43, https://doi.org/ten.3389/fmars.2014.00043 (2014).
-
Marcovaldi, M. A., Godfrey, M. H. & Mrosovsky, North. Estimating sexual practice ratios of loggerhead turtles in Brazil from pivotal incubation durations. Tin can. J. Zool. 75, 755–770 (1997).
-
Laloë, J.-O., Esteban, Northward., Berkel, J. & Hays, G. C. Sand temperatures for nesting sea turtles in the Caribbean: Implications for hatchling sexual activity ratios in the face of climate change. J. Exp. Mar. Bio. Ecol. 474, 92–99, https://doi.org/10.1016/j.jembe.2015.09.015 (2016).
-
Patino‐Martinez, J., Marco, A., Quiñones, 50. & Hawkes, 50. A. A potential tool to mitigate the impacts of climate change to the Caribbean leatherback sea turtle. Glob. Change Biol. 18, 401–411 (2012).
-
Hanson, J., Wibbels, T. & Martin, R. Eastward. Predicted female bias in sex ratios of hatchling loggerhead ocean turtles from a Florida nesting beach. Can. J. Zool. 76, 1850–1861 (1998).
-
Broderick, A. C., Godley, B. J., Reece, S. & Downie, J. R. Incubation periods and sex ratios of light-green turtles: highly female person biased hatchling product in the eastern Mediterranean. Mar. Ecol. Prog. Ser. 202, 273–281 (2000).
-
Fuentes, Thousand. Thousand. P. B. et al. Proxy indicators of sand temperature assist project impacts of global warming on bounding main turtles in northern Australia. Endanger. Species Res. 9, 33–forty, https://doi.org/10.3354/esr00224 (2009).
-
Baptistotte, C., Scalfoni, J. T. & Mrosovsky, Due north. Male-producing thermal ecology of a southern loggerhead turtle nesting beach in Brazil: implications for conservation. Anim. Conserv. 2, 9–xiii (1999).
-
Esteban, North., Laloë, J.-O., Mortimer, J. A., Hays, G. C. & Guzman, A. Male hatchling production in ocean turtles from one of the world's largest marine protected areas, the Chagos Archipelago. Sci. Rep. vi, 20339, https://doi.org/10.1038/srep20339 (2016).
-
Kamel, S. J. Vegetation cover predicts temperature in nests of the hawksbill sea turtle: implications for beach management and offspring sex ratios. Endanger. Species Res. xx, 41–48, https://doi.org/10.3354/esr00489 (2013).
-
Klein, C. J. et al. Prioritization of marine turtle management projects: a protocol that accounts for threats to dissimilar life history stages. Conserv. Lett. x, 547–554, https://doi.org/10.1111/conl.12324 (2017).
-
Rivas, M. 50., Spínola, M., Arrieta, H. & Faife‐Cabrera, 1000. Effect of farthermost climatic events resulting in prolonged precipitation on the reproductive output of sea turtles. Anim. Conserv, https://doi.org/x.1111/acv.12404 (2018).
-
Hill, J. E., Paladino, F. 5., Spotila, J. R. & Tomillo, P. S. Shading and Watering every bit a Tool to Mitigate the Impacts of Climate Change in Sea Turtle Nests. PLoS Ane 10(6), e0129528, https://doi.org/ten.1371/journal.pone.0129528. (2015).
-
Wood, A., Booth, D. T. & Limpus, C. J. Dominicus exposure, nest temperature and loggerhead turtle hatchlings: Implications for beach shading management strategies at sea turtle rookeries. J. Exp. Mar. Biol. Ecol. 451, 105–114, https://doi.org/10.1016/j.jembe.2013.11.005 (2014).
-
Valenzuela, N. C. shift, and natural temperature effects on sex determination in Podocnemis expansa turtles. J. Ecol. 82, 3010–3024, https://doi.org/10.1890/0012-9658(2001)082[3010:CSANTE]ii.0.CO;two (2001).
-
Jourdan, J. & Fuentes, M. M. P. B. Effectiveness of strategies at reducing sand temperature to mitigate potential impacts from changes in environmental temperature on sea turtle reproductive output. Mitig. Adapt. Strateg. Glob. Change 20, 121–133, https://doi.org/10.1007/s11027-013-9482-y (2013).
-
Revuelta, O. et al. Assessing the efficacy of direct conservation interventions: clutch protection of the leatherback marine turtle in the Dominican Republic. Oryx 49, 677–686, https://doi.org/10.1017/S0030605313001488 (2015).
-
Maulany, R. I., Berth, D. T. & Baxter, G. Southward. Emergence success and sex ratio of natural and relocated nests of olive ridley turtles from Alas Purwo National Park, Eastward Java, Indonesia. Copeia 2012, 738–747, https://doi.org/10.1643/CH-12-088 (2012).
-
McElroy, M. L., Dodd, M. Thou. & Castleberry, S. B. Effects of common loggerhead sea turtle nest management methods on hatching and emergence success at Sapelo Isle, Georgia, USA. Chelonian Conserv. Biol. fourteen, 49–55, https://doi.org/10.2744/ccab-14-01-49-55.1 (2015).
-
Chadee, 10. T. & Clarke, R. M. Large-calibration wind energy potential of the Caribbean area region using near-surface reanalysis information. Renew. Sust. Energ. Rev. xxx, 45–58 (2014).
-
Kamel, S. J. & Mrosovsky, Northward. Deforestation: risk of sexual practice ratio distortion in hawksbill bounding main turtles. Ecol. Appl. 16, 923–931 (2006).
-
Foley, A. Yard., Peck, S. A. & Harman, M. R. Effects of sand characteristics and inundation on the hatching success of loggerhead body of water turtle (Caretta caretta) clutches on low-relief mangrove islands in southwest Florida. Chelonian Conserv. Biol. v, 32–41 (2006).
-
Refsnider, J. M., Warner, D. A. & Janzen, F. J. Does shade cover availability limit nest-site choice in 2 populations of a turtle with temperature-dependent sex determination? J. Therm. Biol. 38, 152–158, https://doi.org/10.1016/j.jtherbio.2013.01.003 (2013).
-
Miller, J. D. Reproduction in sea turtles. Pages 65–96 in P. 50. Lutz and J. A. Musick (editors), The Biology of Body of water Turtles, Volume I. CRC Press, Boca Raton, Florida, The states. (1997).
-
Katselidis, K. A., Schofield, G., Stamou, G., Dimopoulos, P. & Pantis, J. D. Employing sea-level rise scenarios to strategically select sea turtle nesting habitat important for long-term management at a temperate breeding area. J. Exp. Mar. Bio. Ecol. 450, 47–54, https://doi.org/ten.1016/j.jembe.2013.10.017 (2014).
-
Fuentes, 1000. 1000. P. B., Limpus, C. J. & Hamann, M. Vulnerability of bounding main turtle nesting grounds to climate change. Global Change Biol. 17, 140–153 (2011).
-
Fuentes, M. M. P. B. & Porter, West. P. Using a microclimate model to evaluate impacts of climate change on ocean turtles. Ecol. Model. 251, 150–157, https://doi.org/10.1016/j.ecolmodel.2012.12.020 (2013).
-
Lopez, K. G. et al. Coastal development at sea turtles nesting ground: Efforts to constitute a tool for supporting conservation and littoral direction in northeastern Brazil. Ocean Coast. Manage. 116, 270–276, https://doi.org/10.1016/j.ocecoaman.2015.07.027 (2015).
-
Laloë, J.-O., Cozens, J., Renom, B., Taxonera, A. & Hays, Grand. C. Climatic change and temperature‐linked hatchling bloodshed at a globally of import body of water turtle nesting site. Glob. Alter Biol. 23, 4922–4931, https://doi.org/10.1111/gcb.13765 (2017).
-
Wedekind, C. Managing Population Sex Ratios in Conservation Practice: How and Why? In T. Pivilitis (editor) Topics in Conservation Biology, ISBN: 978-953-51-0540-4, InTech. Downloaded from: http://www.intechopen.com/books/topics-in-conservation-biology/managing-population-sexual activity-ratio-why-and-how on28 March 2018 (2012).
-
Girondot, K., Fouillet, H. & Pieau, C. Feminizing turtle embryos as a conservation tool. Conserv. Biol. 12, 353–362 (1998).
-
Houghton, J. D. R. et al. Protracted rainfall decreases temperature within leatherback turtle (Dermochelys coriacea) clutches in Grenada, West Indies: ecological implications for a species displaying temperature dependent sex determination. Journal of experimental marine biology and ecology 345, 71–77, https://doi.org/10.1016/j.jembe.2007.02.001 (2007).
-
Beger, M. et al. Integrating regional conservation priorities for multiple objectives into national policy. Nat. Commun. 6, 8208, https://doi.org/10.1038/ncomms9208 (2015).
-
Cantarelli, V. H., Malvasio, A. & Verdade, L. M. Brazil'southward Podocnemis expansa conservation program: retrospective and future directions. Chelonian Conserv. Biol. thirteen, 124–128 (2014).
-
Esteban, Northward., van Dam, R. P., Harrison, E., Herrera, A. & Berkel, J. Green and hawksbill turtles in the Bottom Antilles demonstrate behavioural plasticity in inter-nesting behaviour and post-nesting migration. Mar. Biol. 162, 1153–1163, https://doi.org/x.1007/s00227-015-2656-ii (2015).
-
R Core Team. R: A language and surroundings for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. www.R-project.org (2014).
-
Bates, D., Maechler, Thousand., Bolker, B. & Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 67, i–48, https://doi.org/10.18637/jss.v067.i01 (2015).
Acknowledgements
We thank students and volunteers that supported Due south.U., F.S.P.L.K. and J.B. during experimental piece of work. Experimental blueprint fieldwork by Northward.E. was supported by IMARES. 1000.J.A.C. and 50.B. were supported through the project "Ecology and conservation of green and hawksbill turtles in the Dutch Caribbean" funded by kingdom of the netherlands Organization of Scientific Inquiry (NWO-ALW 858.fourteen.090). Fieldwork of F.S.P.50.One thousand. was supported past the FONA foundation and Alberta Mennega Stichting. The authors admit the use of the Maptool program (www.seaturtle.org) for the product of Figure 2.
Writer information
Affiliations
Contributions
North.E. and M.J.A.C. conceived the study. N.E., E.H.M. and S.U. developed the experimental shading design and S.U. carried out fieldwork. N.Due east., M.J.A.C., Fifty.Due east.B. and F.S.P.50.K. developed the experimental design to exam drivers of temperature and F.S.P.Fifty.G. conducted fieldwork. J.B. supported all fieldwork. J.-O.L. led the data assay with assistance from N.E. and 1000.C.H. N.E. led the writing of the manuscript with contributions from all authors.
Respective author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open up Admission This article is licensed under a Artistic Commons Attribution 4.0 International License, which permits use, sharing, accommodation, distribution and reproduction in any medium or format, as long as you requite appropriate credit to the original author(due south) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party cloth in this commodity are included in the article's Creative Eatables license, unless indicated otherwise in a credit line to the textile. If fabric is not included in the commodity's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted employ, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this commodity
Esteban, N., Laloë, JO., Kiggen, F.Due south.P.L. et al. Optimism for mitigation of climate warming impacts for ocean turtles through nest shading and relocation. Sci Rep viii, 17625 (2018). https://doi.org/10.1038/s41598-018-35821-6
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/ten.1038/s41598-018-35821-6
Keywords
- Artificial Shade
- Decreasing Incubation Temperature
- Rookeries
- Nest Relocation
- Turtle Populations
Farther reading
Comments
By submitting a annotate you lot agree to bide past our Terms and Community Guidelines. If you find something abusive or that does non comply with our terms or guidelines please flag information technology as inappropriate.
What Are Scientists Most Concerned About With Respect To Sea Turtle Conservation And Climate Change?,
Source: https://www.nature.com/articles/s41598-018-35821-6
Posted by: medranosookinium.blogspot.com


0 Response to "What Are Scientists Most Concerned About With Respect To Sea Turtle Conservation And Climate Change?"
Post a Comment